There’s the genome, the transcriptome, the microbiome… and now the NLRome?
Breeders and pathologists have long been interested in uncovering the secrets of plant immunity, and much of their attention has been focused on receptors that can activate immune signalling: cell-surface proteins that recognize microbe-associated molecular patterns (MAMPs), and intracellular proteins that detect pathogen effectors, including nucleotide-binding leucine-rich repeat receptors (NLRs).
Hundreds of NLR genes can be found in the genomes of flowering plants. They are believed to form inflammasome-like structures, or resistosomes, that control cell death following pathogen recognition, and are being investigated as candidates for engineering new pathogen resistances.
For these reasons, scientists are keen to create inventories of NLR genes at different taxonomic levels. But their efforts have been hindered by the extraordinarily polymorphic nature of the gene family, patterns of allelic and structural variation, and clusters with extensive copy-number variation.
Two research teams have successfully overcome these challenges by combining resistance gene enrichment sequencing (RenSeq) with SMRT Sequencing.
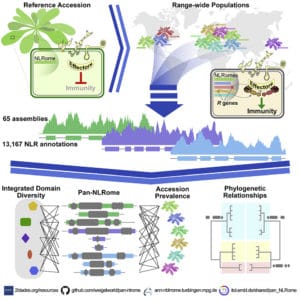
In a recent paper in Cell, a multi-institutional team led by Felix Bemm and colleagues at the Max Planck Institute for Developmental Biology in Germany detailed their creation of a nearly complete species-wide pan-NLRome in Arabidopsis thaliana.
The sequences they obtained allowed them to define the core NLR complement, as well as to chart integrated domain diversity, describe new domain architectures, assess presence or absence of polymorphisms in non-core NLRs, and map uncharacterized NLRs onto the A. thaliana Col-0 reference genome.
“Reference genomes likely include only a fraction of distinct NLR genes within a species, which in turn has made it impossible to obtain a clear picture of NLR diversity based on resequencing efforts,” the authors wrote.
“Our work provides a foundation for the identification and functional study of disease-resistance genes in agronomically important species with more complex genomes,” they added.
Using RenSeq to trace NLR evolution in tomatoes
Another team from the University of California at Berkeley, led by Brian Staskawicz and first author Kyungyong Seong, explored the NLRome of the tomato plant Solanum lycopersicum.
This important crop is challenged by more than 200 diseases caused by diverse pathogens. Low genetic diversity and changes of resistance (R) genes of the cultivated tomato during domestication have led to a heightened urgency for genetic improvement. Scientists are keen to draw upon the hereditary disease resistance of wild tomato species that have co-evolved with their pathogens in highly diverse habitats, and R genes in particular, which have shown more durable resistance against multiple pathogens.
Previous attempts to use RenSeq to selectively capture and sequence NLRs in tomato have been limited by the method’s inability to completely resolve highly repetitive sequences and physical clusters of NLRs, the team noted in a bioRxiv preprint, so they turned to SMRT Sequencing as well.
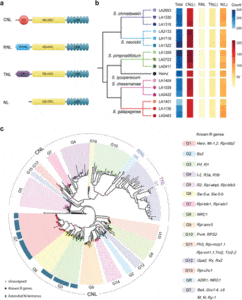
They employed SMRT RenSeq to identify NLRs from 18 Solanaceae accessions (S. lycopersicum Heinz, Nicotiana benthamiana, Capsicum annuum ECW20R, plus 15 wild tomato accessions belonging to five species).
They produced 264 to 332 high-quality NLR gene models in tomato, and annotated 314 NLRs alongside the reference genome of S. lycopersicum Heinz.
“Our RenSeq results improved the annotation of 128 NLRs, including 13 existing annotations which were incomplete because of mis-assembly or unfilled gaps,” the authors wrote.
“We demonstrated that SMRT RenSeq is a cost-effective, efficient alternative to the whole genome sequencing. We also verified that SMRT RenSeq was capable of…resolving the complexity of NLRs and their clusters.”
The team used the gene models and annotations to explore NLR evolution, but noted that larger scale comparative studies including evolutionarily distant wild tomato species should be done to provide more comprehensive insights, and to expand the scope of NLRome for genome engineering and breeding of tomatoes.
“Our study provides high quality gene models of NLRs that can serve as resources for future studies for crop engineering and elucidates greater evolutionary dynamics of the extended NLRs than previously assumed,” the authors wrote.
November 11, 2019 | Plant + animal biology